The drug Hertraz 440 has the main ingredient trastuzumab, which is prepared in the form of lyophilized powder for injection. This drug belongs to the group of anticancer drugs, monoclonal antibodies.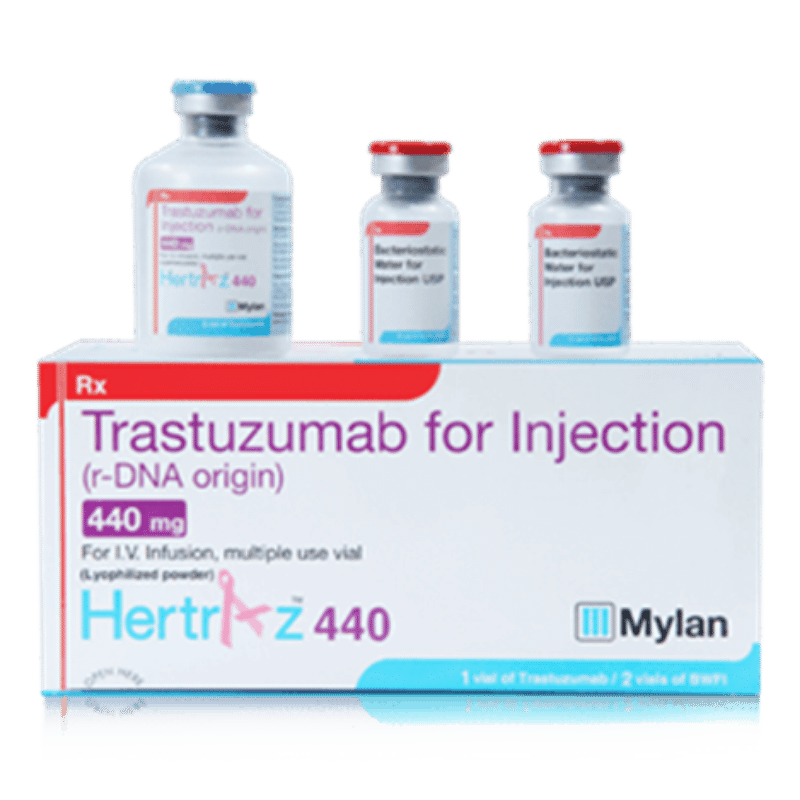
- Country: India
- Brand: RV Healthcare
1. What are the uses of Hertraz 440?
In the human body, HER2 is a gene that is present and plays a role in the development of breast cancer and some cases of stomach cancer. Normally, the HER2 gene makes HER2 proteins, which are receptors present on breast cells that control the normal functioning of the mammary glands. However, when the HER2 genes are mutated, they work incorrectly and make too many copies (HER2 amplification). Up to 1⁄5 breast cancers are identified with HER2 amplification. In addition, there are some cases of stomach cancer also stem from this factor.
The active ingredient trastuzumab contained in Hertraz 440 is a recombinant IgG1 monoclonal antibody that binds with extracellular HER2 affinity. It was this association that inhibited HER2 signaling independently of the ligand, while also preventing extracellular cleavage. This results in inhibition of tumor cell proliferation.
Based on the above mechanism, Hertraz 440 is often prescribed by doctors in cases such as:
Metastatic breast cancer with tumor overexpressing HER2: Can be used as monotherapy or in combination with piclitaxel, decetaxel, and aromatase inhibitors.
HER2-positive early breast cancer: Can be used with chemotherapy, radiotherapy or in combination with drugs such as piclitaxel and decetaxel.
HER2-positive metastatic gastric cancer: used in combination with cabecita battery or 5-fluorouracil and cisplatin.
2. Dosage and how to use Hertraz 440
Hertraz 440 is recommended to be administered to patients weekly or every 3 weeks depending on the condition.
The recommended weekly Hertraz 440 infusion schedule is as follows:
Loading dose: The initial loading dose is usually 4mg/kg, given intravenously over 90 minutes. The patient should be monitored during the infusion because some symptoms may appear such as fever, chills.
Subsequent dose: The recommended weekly dose is 2mg/kg body weight. Treatment should be continued until the patient improves.
The recommended 3-weekly infusion schedule of Hertraz 440 is as follows:
Patients were initiated on a loading dose of 8 mg/kg body weight. 3 weeks after the dose of 6mg/kg. Repeat every 3 weeks the dose of 6mg/kg. Intravenous infusion over 90 minutes, if well tolerated by the patient can shorten the time to 30 minutes.
For metastatic breast cancer and metastatic gastric cancer, treatment with Hertraz 440 is recommended until the patient has progressed. For early breast cancer, it is necessary to treat for 1 year until the disease recurs before starting again.
Hertraz 440 should be administered intravenously. Absolutely do not use the drug by rapid intravenous injection or rapid infusion.
Because Hertraz 440 is packaged as a vial containing powder for injection. Therefore, the use of drugs must be under the supervision and support of medical staff.
3. Undesirable effects of the drug Hertraz
Since Hertraz 440 is an anticancer drug, patients tend to experience many side effects. Some undesirable effects of Hertraz 440 may include:
Increased likelihood of bacterial and parasitic infections: Patients are prone to diseases such as pneumonia, cystitis, shingles, sinusitis, skin infections and cellulitis,...
Blood and lymphatic system disorders: Fever, leukopenia,...
Immune system disorders: Hypersensitivity reactions and anaphylaxis,...
Nutritional disorders: loss of appetite, loss of taste, weight loss,...
Psychiatric disorders: Anxiety, insecurity, insomnia, depression,...
Nervous system disorders: Tremor, dizziness, paresthesia and hypertonia.
Cardiac Disorders: Increased or decreased blood pressure, erratic heartbeat, palpitations, palpitations and arrhythmias,...
Musculoskeletal disorders: arthralgia, myalgia, muscle tension, pain in extremities and muscle spasms,...
Local reactions to the intravenous infusion of Hertraz 440: chills, fatigue, pain, edema and discomfort,...
4. Precautions and warnings when using Hertraz 440
When treating with Hertraz 440, patients should note a few things as follows:
- Patients should be tested for HER2 before receiving treatment with Hertraz 440.
- Do not dilute Hertraz 440 with glucose solution because of the risk of precipitation.
- Attention should be paid to monitoring clinical reactions during the infusion of the patient. If undesirable manifestations such as fever, hypotension, arrhythmia, anaphylaxis, etc. occur, the infusion rate should be reduced or the infusion discontinued.
- The drug should be stored at a temperature of 2 - 8 degrees C. Avoid sunlight.
- Patients treated with Hertraz 440 may experience cardiac dysfunction or an increased risk of congestive heart failure. Therefore, it is necessary to carefully monitor the health of these patients.